Identify the Substance With the Highest Viscosity.
Substance ViscosityCP Mercury 153 Water 089 Kerosene 164 Glycerine 950 So glycerine has the greatest viscosity. Choose the substance with the highest viscosity A.

What S The Matter Matter Is Everything Around You The Earth Is One Big Mixture Of Gases Liquids And Solids All Of Which Are Different Forms Of Matter Ppt Download
Honey syrup glue High viscosity means that the thickness of the material is very thick compared to low viscosity which is rather thin.

. Water at 40 C B. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. Liquids like water have a much lower viscosity compared to liquids such as honey.
Choose the substance with the highest viscosity. Which would have the highest viscosity. Water at 40 C B.
Glass is actually a supercooled liquid and would have a very high viscosity if you include supercooled liquids. It is one of the most viscous organic substances that can be found in nature. Examples of substances that has high viscosity.
Consider the following molecules. CH 3CH 3 CH 3OH and CH 3CH 2OH Answers. C corn syrup 36 Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
C HOCH 2 CH 2. Viscosity is governed by the strength of intermolecular forces and especially by the shapes of the molecules of a liquid. A CH3 CH 2 2 CO.
Water at 10 C D. Water at 50 C. Exam 2 1412 Practice1docx.
Water at 10 C. Hot water room temperature water room temperature olive oil hot olive oil. Science Chemistry QA Library Choose the substance with the highest viscosity A.
35 Identify the substance with the highest viscosity. A table of common liquids grouped by class or type including information on viscosity at a given temperature whether the liquid is Newtonian or Thixotropic. Honey Glues Peanut butter Long hydrocarbon chain Ketchup and many more.
The degrees of viscosity in alcohols are usually very low. Which substance has the highest viscosity. Identify the substance with the highest viscosity.
A CH 3CH 3 has only dispersion forces whereas the other two substances have both dispersion forces and hydrogen bonds. Also called bitumen it is a mixture of black organic substances that contain a high density completely soluble incarbon disulfideand it is composed mainly by hydrocarbons. Larger molecules usually have higher viscositys than analogous smaller molecules.
A diesel B water C maple syrup D motor oil E coffee. More dramatically a long-chain hydrocarbon like squalene C 30 H 62 has a viscosity an order of magnitude larger than the shorter n-alkanes. Indicate a plan on how you intend to solve the problem.
Na2O HCl NH3 N2 CH3CH2OH. In fact some people dont even realize peanut butter is a liquid because it looks like its not flowing at all - but it is - just very slowly. The fluid that has high viscosity will flow slowly whereas the fluid having low viscosity will flow faster comparatively.
We have to remember that the viscosity depends upon the flowing capability of a fluid. It also depends on temperature. The opposite of viscosity is fluidity which measures the ease of flow while liquids such as motor oil or honey which are sluggish and high in viscosity are known as viscousOne may ask the question of what is actually going on in the.
Water at 20 C C. Water at 20 C C. Choose the substance with the highest viscosity.
In general as temperature increases for a given substance viscosity decreases. Follow below steps to answer the question. Liquids which flow very slowly like glycerin or honey have high viscosities.
AIdentify the intermolecular forces present in the following substances and B select the substance with the highest boiling point. This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. Those like ether or gasoline which flow very readily have low viscosities.
B C 2 H 4 Cl 2. C2H4Br2 CH3CH22CO HOCH2CH2CH2CH2OH C8H18 CI4. You should have a numerical list.
Of the four liquids listed in the last section peanut butter is the thickest - and it has the highest viscosity because it will flow the slowest. B CH 3CH 2OH. At high temperatures when it is workable glass has a relatively low viscocity but at room temperature its viscosity is huge something around the area of 1035 poise IIRC.
Water at 30 C E. Viscosity can be not only a fluids resistance to flow but also a gas resistance to flow change shape or movement. A great example is glass.
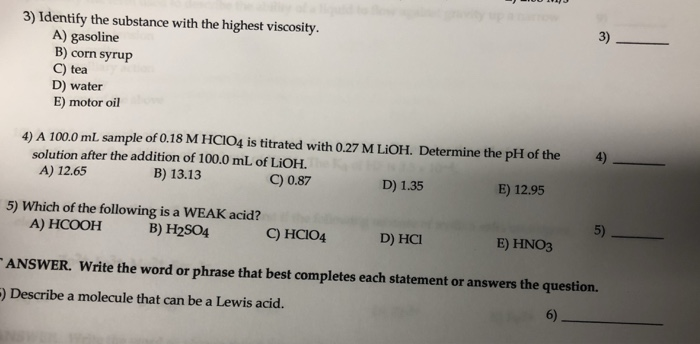
Solved 3 Identify The Substance With The Highest Viscosity Chegg Com

Choose The Substance With The Lowest Viscosity In 2022 Viscosity Covalent Bonding Substances
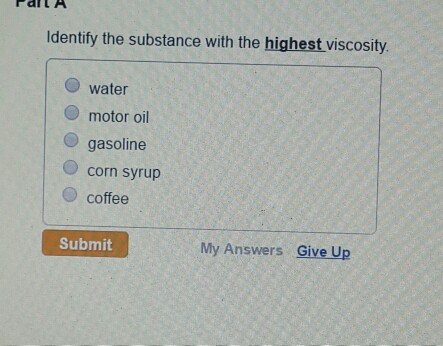
Solved Identify The Substance With The Highest Viscosity Chegg Com

Chapter 11 Intermolecular Forces Liquids And Solids Ppt Download
0 Response to "Identify the Substance With the Highest Viscosity."
Post a Comment